2.Specification:98%
3.CAS:33069-62-4
4.Free Sample
5.Capsules,tablets,softgel,gummies,small bags,private label
6.Test Method:HPL,UV&GC
7.Vacuum Aluminum Foil Bag; Fiber Drum for 25kg with inside double layer food grade poly bag
8.DeliveryTime:1-3 working days
Organic,Gluten free,no Allergen,Non-GMO,Paraben free,No Colors, BSE/TESE Free,Vegan
- Product Description
Paclitaxel Powder Manufacturer and Supplier
At Shaanxi Fairir Biotech Co., Ltd. we pride ourselves on being a leading manufacturer and supplier of premium Paclitaxel Powder. Our state-of-the-art facilities and rigorous quality control processes ensure that we deliver the highest grade API for pharmaceutical companies worldwide. With our extensive experience in plant extraction and advanced technologies, we offer product that meets the strictest industry standards.

What is Paclitaxel Powder?
The product is a potent chemotherapy agent derived from the bark of the Pacific yew tree. It's widely used in the treatment of various cancers, including ovarian, breast, lung, and pancreatic cancer. As a crucial component in oncology drug formulations, our high-purity product ensures optimal efficacy and safety in pharmaceutical applications.
Why Choose Us?
- GMP-certified production facility
- Advanced extraction and purification technologies
- Comprehensive quality management system
- Customizable solutions for your specific needs
- Reliable supply chain with global logistics support
COA (Certificate of Analysis)
|
TEST |
TEST METHOD |
SPECIFICATIONS |
RESULT |
|
|
ASSAY (calculated on the anhydrous, solvent-free basis) |
USP<621> |
97.0%~102.0% |
100.40% |
|
|
Appearance |
USP<621> |
White or almost white crystalline powder. |
White crystalline powder |
|
|
SPECIFIC ROTATION |
USP<781S> Method Ic |
-49.0°~-55.0° |
-52.1° |
|
|
IDENTIFICATION
|
IR USP<197K> |
Infrared Absorption:In accordance with the reference Standard spectrum of paclitaxel |
Conforms |
|
|
HPLC USP<621> |
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay. |
Conforms |
||
|
(HPLC) RELATED COMPOUNDS
|
10-Deacetylbaccatin Ⅲ |
USP<621>Test
|
NMT 0.1% |
Undetected |
|
Baccatin III |
NMT 0.2% |
Undetected |
||
|
Photodegradant |
NMT 0.1% |
Undetected |
||
|
10-Deacetylpaclitaxel |
NMT 0.5% |
Undetected |
||
|
2-Debenzoylpaclitaxel-2-pentenoate |
NMT 0.1% |
0.03% |
||
|
Oxetane ring opened, acetyl and benzoyl migrated |
NMT 0.4 |
Undetected |
||
|
10-Acetoacetylpaclitaxel |
||||
|
10-Deacetyl-7-epipaclitaxel |
||||
|
7-Epipaclitaxel |
NMT 0.4% |
≤0.03% |
||
|
10,13-Bissidechainpaclitaxel |
NMT 0.5% |
Undetected |
||
|
7-Acetylpaclitaxel |
NMT 0.6% |
Undetected |
||
|
13-Tes-baccatin III |
NMT 0.1% |
Undetected |
||
|
7-Tes-paclitaxel |
NMT 0.3% |
Undetected |
||
|
Others |
NMT 0.1% (each) |
0.03% |
||
|
Total |
NMT 2.0% |
0.14% |
||
|
ORGANIC VOLATILE IMPURITIE
|
Acetone |
|
NMT 5000ppm |
26ppm |
|
n-heptane |
|
NMT 5000ppm |
2102ppm | |
|
Dichloromethane |
|
NMT 600ppm |
Undetected |
|
|
Ethanol |
|
NMT 5000ppm |
20ppm |
|
|
WATER |
USP <921> Method Ic |
NMT 2.0% |
0.66% |
|
|
RESIDUE ON IGNITION |
USP<281> |
NMT 0.2% |
0.05% |
|
|
HEAVY METALS |
USP<231>Method Ⅱ |
NMT 0.001% |
Conforms |
|
|
BACTERIAL ENDOTOXINS |
USP<85> |
NMT 0.4 EU/mg |
Conforms |
|
|
MICROBIAL LIMITS
|
USP<61>
|
Total aerobic microbial count NMT100 cfu/g |
10 cfu/g |
|
|
It meets the requirements of the tests for the absence of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella species, and Escherichia coli |
Conforms |
|||
|
CONCLUSION |
The product is in accordance with USP43to test the above-mentioned items, the test results conform to the specification. |
|||
*Please contact us for the latest COA.
Paclitaxel Powder Function
Paclitaxel functions as a mitotic inhibitor, disrupting normal cell division by stabilizing microtubules. This mechanism of action makes it highly effective in preventing the growth and spread of cancer cells, making it a cornerstone in many chemotherapy regimens.
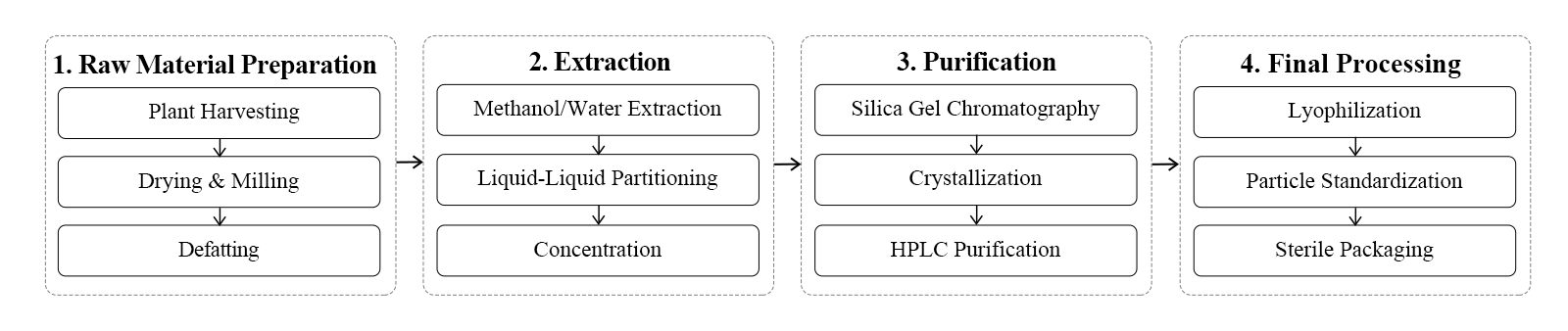
Our Manufacturing Process
Our production of the product involves a meticulous process that combines traditional extraction methods with cutting-edge technology. We begin with carefully sourced yew bark, which undergoes our proprietary extraction process. This is followed by multiple purification steps, including chromatography and recrystallization, to achieve the highest purity levels. Our advanced quality control measures ensure consistent potency and purity in every batch.
Benefits for Global Buyers
- Consistent High Quality: Our stringent quality control measures ensure batch-to-batch consistency, meeting or exceeding pharmacopoeial standards.
- Regulatory Compliance: We provide comprehensive documentation to support your regulatory filings, including DMF access upon request.
- Flexible Ordering: We offer various pack sizes and can accommodate both small-scale R&D needs and large-volume commercial orders.
- Technical Support: Our expert team is available to provide formulation guidance and analytical method support.
- Competitive Pricing: Our efficient production processes allow us to offer competitive pricing without compromising on quality.
- Supply Chain Security: With our robust supply chain management, we ensure timely deliveries and maintain buffer stocks for key customers.
- Sustainability: We employ environmentally friendly extraction methods and adhere to responsible sourcing practices.
Paclitaxel Powder Applications
Paclitaxel Powder is primarily used in the pharmaceutical industry for the formulation of injectable cancer treatments. It's a key ingredient in various drug delivery systems, including nanoparticle albumin-bound (nab) formulations and liposomal preparations. Our high-purity powder is also suitable for research purposes, enabling scientists to explore new applications and delivery methods for this potent compound.
Quality Assurance
Our commitment to quality is unwavering. We employ a multifaceted approach to ensure the purity and efficacy of our product:
- In-process controls at every production stage
- Advanced analytical techniques including HPLC, GC, and mass spectrometry
- Stability testing under various conditions
- Microbial and endotoxin testing
Certification
- ISO 9001:2015
- GMP Certified
- HACCP
- Kosher
- Halal
OEM/ODM Services
We offer comprehensive OEM/ODM services, including custom synthesis, formulation development, and packaging solutions tailored to your specific requirements.

Packaging and Delivery
Our product is available in various pack sizes, from gram quantities to bulk kilograms. We use specialized, temperature-controlled packaging to ensure product stability during transit. Our global logistics network ensures timely delivery to any location worldwide.

FAQ
- Q: What is the purity of your product?
A: Our standard purity is ≥99%, with higher grades available upon request. - Q: Do you provide samples?
A: Yes, we offer samples for evaluation purposes. Please contact our sales team for details. - Q: What is the minimum order quantity?
A: Our MOQ is typically 1 gram, but we're flexible based on your specific needs. - Q: How do you ensure the stability of Paclitaxel during shipping?
A: We use specialized cold-chain logistics and temperature-monitored packaging to maintain product integrity. - Q: Can you assist with regulatory documentation for drug applications?
A: Absolutely. We provide comprehensive technical dossiers and can support your regulatory filings. - Q: What is your production capacity for the product?
A: We can produce up to 100 kg annually, with the ability to scale up based on demand.
Contact Us
For inquiries about our Paclitaxel Powder or to discuss your specific requirements, please contact our sales team at sales@fairirbiotech.com. We're committed to providing you with the highest quality products and exceptional service to support your pharmaceutical manufacturing needs.



















_1751965378790.webp)